Abstract

Chimeric antigen receptor (CAR)-expressing T cells targeting B-cell maturation antigen (BCMA) are effective multiple myeloma therapy, but improvement in durability of responses is needed. We incorporated a fully-human heavy-chain-only anti-BCMA binding domain (FHVH33) into a CAR. Since it is only a heavy chain domain with no linker or artificial junctions, FHVH33 should be less immunogenic than a scFv. The CAR containing FHVH33 is called FHVH33-CD8BBZ. FHVH33-CD8BBZ contains CD8α hinge and transmembrane domains, a 4-1BB domain, and a CD3ζ domain; it was encoded by a γ-retroviral vector (Lam et al. Nature Communications 2020). T cells expressing FHVH33-CD8BBZ (FHVH-T) were produced from unsorted blood mononuclear cells in 7 days.
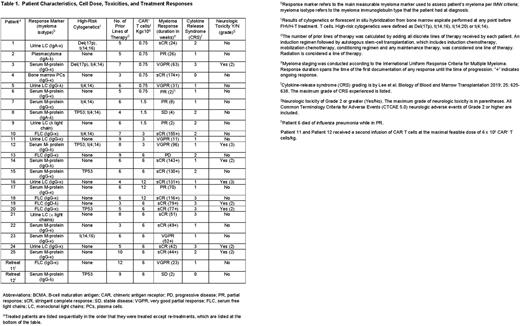
Twenty-five patients received 300 mg/m2 of cyclophosphamide and 30 mg/m2 of fludarabine on days -5 to -3 followed by infusion of FHVH-T on day 0. Five dose levels were assessed, and 6x106 CAR+ T cells/kg was selected for an expansion cohort based on efficacy and manufacturing factors (Table). Patients had received a median of 6 lines of therapy (range 3 to 10) prior to enrollment. Twenty-three of 25 patients (92%) obtained partial response (PR) or better. Eighteen patients (72%) attained best responses of stringent complete response (sCR) or very good partial response (VGPR). Median progression-free survival (PFS) of all treated patients is 65 weeks to date; eleven patients have ongoing responses.
One striking feature of the anti-myeloma activity of FHVH-T was the rapidity of myeloma elimination. Some myeloma markers including serum free light chains, urine monoclonal light chains, and bone marrow plasma cells decrease rapidly after any type of effective therapy while intact immunoglobulin monoclonal proteins decrease slowly due to long serum half-lives, which are 20-25 days for most types of IgG. Following rapidly changing myeloma markers such as free light chains shows the actual tempo of myeloma elimination without the confounding factor of long serum half-lives of intact immunoglobulins. Among the 12 patients with rapidly changing main myeloma markers, most anti-myeloma activity occurred within the first month after FHVH-T infusion since myeloma markers underwent a 99-100% reduction in all 12 patients by 1 month after FHVH-T infusion. In 10/12 patients with rapidly changing main myeloma markers, a 99-100% decrease had occurred by 14 days after FHVH-T infusion.
We assessed FHVH-33 T early after infusion during the time of most anti-myeloma activity. Blood CAR+ cell levels were assessed by quantitative PCR (qPCR). The median peak blood CAR+ cell number was 121 (range 3 to 3414) cells/μL. Peak CAR+ cell levels occurred a median of 12 (range 7 to 15) days after FHVH-T infusion. One month after infusion, the median blood CAR+ cell number was 11 (range 0 to 305) cells/μL. Median blood CAR+ cell levels one month after infusion were 20 cells/μL in patients with a PFS of 12 months or more versus 4 cells/μL in patients with PFS less than 12 months (P=0.0273).
The CD4/CD8 ratio of CAR+ T cells decreased from the time of infusion until the time of peak blood CAR+ cells (P=0.0004). From the time of infusion until the time of peak blood CAR+ cells, there was an increase the percentage of T cells with an effector memory phenotype (C-C-chemokine receptor-7 (CCR7)-negative and CD45RA-negative), (P<0.001). Expression of CCR7 on infusion CD4+ FHVH-T correlated with peak CAR+ cell level (P=0.0018). Frequency of infusion CD4+ FHVH-T with a central memory phenotype (CCR7+, CD45RA-negative) correlated with peak and 1-month post-infusion CAR+ cell levels.
Peak interferon (IFN)γ and interleukin (IL)-6 levels were associated with Grade 3 or 4 CRS (P=0.0034). Peak serum levels of IFNγ, IL2, IL6, IL8, IL10, and IL15 were higher (P<0.003 for all) in patients with neurologic toxicity. Single-cell RNA sequencing revealed extensive changes in FHVH-T gene expression between infusion and the time of peak blood CAR+ cell levels.
Surprisingly, humoral immune responses against the fully-human CAR expressed by FHVH-T were detected in 4/12 evaluated patients after one FHVH-T infusion. In one patient who received 2 FHVH-T treatments, a humoral anti-CAR response was associated with lack of efficacy and absence of blood FHVH-T.
FHVH-T therapy led to durable responses with most anti-myeloma activity occurring within 14 days after FHVH-T infusion. Blood levels of FHVH-T correlated with CD4+CCR7+ infusion cells.
Disclosures
Brudno:Kyverna Therapeutics, Inc.: Other: unpaid member of scientific advisory board. Goff:Clinigen: Other: In-kind support (Drug for Clinical Trial). Lam:Kite, a Gilead Company: Patents & Royalties. Kochenderfer:Kyverna Therapeutics, Inc: Patents & Royalties; Bristol Myers Squibb: Patents & Royalties, Research Funding; Kite, a Gilead Company: Patents & Royalties, Research Funding.
OffLabel Disclosure:
cyclophosphamide 300 mg/m2 fludarabine 30 mg/m2 Conditioning chemotherapy prior to CAR T-cell infusion
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal